Chemistry Question Calculate the ratio of effusion rates for nitrogen (N_2) (N 2) and neon (Ne). Solutions Verified Solution A Solution B Solution C Answered 1 month ago Create an account to view solutions Recommended textbook solutions Chemistry: Matter and Change 1st Edition • ISBN: 9780078746376 (2 more) Buthelezi, Dingrando, Hainen, Wistrom
30 Day Watercolor Challenge – Learn to Paint 30 Nature Landscapes With Watercolor | Umashree Taparia | Skillshare
Solution A Solution B Create an account to view solutions Recommended textbook solutions Pearson Chemistry Matta, Staley, Waterman, Wilbraham 3,748 solutions Chemistry: The Central Science 14th Edition Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown 7,848 solutions

Source Image: m.youtube.com
Download Image
Chemistry Chemistry questions and answers Calculate the ratio of effusion rates for nitrogen (N) and neon (Ne). Calculate the ratio of diffusion rates for carbon monoxide and carhon dioxide This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: opensea.io
Download Image
Calculate the ratio of effusion rates for nitrogen $(N_2)$ a | Quizlet Example 1: Applying Graham’s Law to Rates of Effusion. Calculate the ratio of the rate of effusion of hydrogen to the rate of effusion of oxygen. [reveal-answer q=”190867″]Show Answer[/reveal-answer] [hidden-answer a=”190867″] From Graham’s law, we have: Using molar masses: Hydrogen effuses four times as rapidly as oxygen. [/hidden

Source Image: bartleby.com
Download Image
Calculate The Ratio Of Effusion Rates For Nitrogen And Neon
Example 1: Applying Graham’s Law to Rates of Effusion. Calculate the ratio of the rate of effusion of hydrogen to the rate of effusion of oxygen. [reveal-answer q=”190867″]Show Answer[/reveal-answer] [hidden-answer a=”190867″] From Graham’s law, we have: Using molar masses: Hydrogen effuses four times as rapidly as oxygen. [/hidden Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to
Answered: Calculate the relative effusion rates… | bartleby
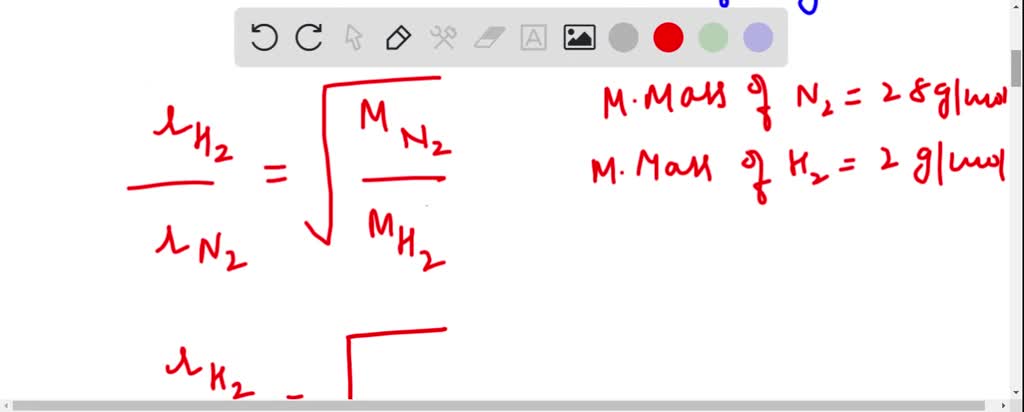
Oct 27, 2022Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to SOLVED: Calculate the ratio of the effusion rate of hydrogen gas (H2) to that of nitrogen gas (N2). Express your answer in decimal form and calculate your answer to at least four
Source Image: numerade.com
Download Image
SOLVED: calculate the ratio of effusion rates for n2 and ne Oct 27, 2022Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to
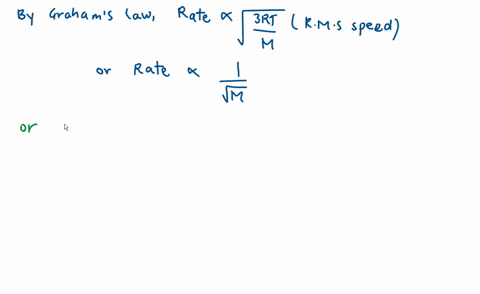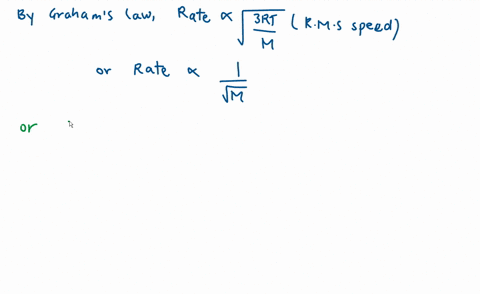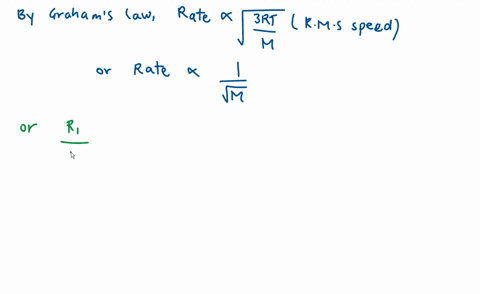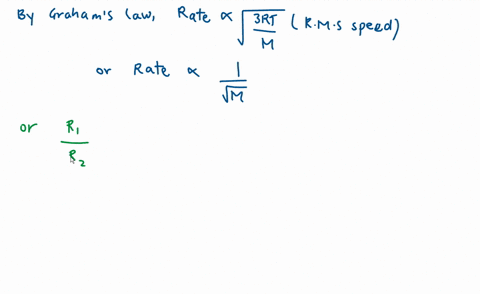
Source Image: numerade.com
Download Image
30 Day Watercolor Challenge – Learn to Paint 30 Nature Landscapes With Watercolor | Umashree Taparia | Skillshare Chemistry Question Calculate the ratio of effusion rates for nitrogen (N_2) (N 2) and neon (Ne). Solutions Verified Solution A Solution B Solution C Answered 1 month ago Create an account to view solutions Recommended textbook solutions Chemistry: Matter and Change 1st Edition • ISBN: 9780078746376 (2 more) Buthelezi, Dingrando, Hainen, Wistrom

Source Image: skillshare.com
Download Image
Calculate the ratio of effusion rates for nitrogen $(N_2)$ a | Quizlet Chemistry Chemistry questions and answers Calculate the ratio of effusion rates for nitrogen (N) and neon (Ne). Calculate the ratio of diffusion rates for carbon monoxide and carhon dioxide This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
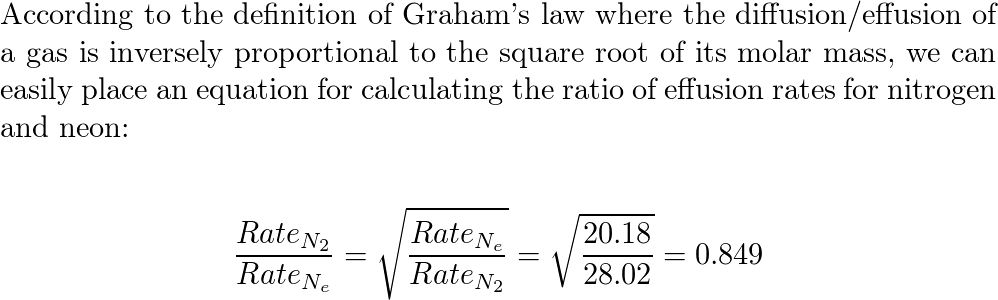
Source Image: quizlet.com
Download Image
⏩SOLVED:Calculate the ratio of effusion rates for nitrogen (N2) and… | Numerade By calling neon “gas 1” and argon “gas 2,” we can compare the effusion rates of the two gases by plugging their molecular masses into the equation. This proportion is equal to the rate of neon effusion over the rate of argon effusion, giving the ratio of neon atoms to argon atoms in container B.

Source Image: numerade.com
Download Image
Quantitatively compare the rates of effusion for the followi | Quizlet Example 1: Applying Graham’s Law to Rates of Effusion. Calculate the ratio of the rate of effusion of hydrogen to the rate of effusion of oxygen. [reveal-answer q=”190867″]Show Answer[/reveal-answer] [hidden-answer a=”190867″] From Graham’s law, we have: Using molar masses: Hydrogen effuses four times as rapidly as oxygen. [/hidden
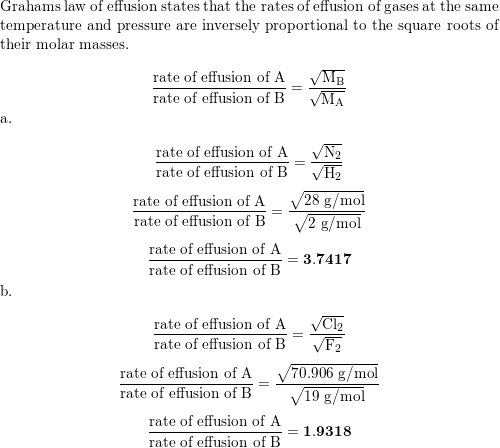
Source Image: quizlet.com
Download Image
⏩SOLVED:Quantitatively compare the rates of effusion for the… | Numerade Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to
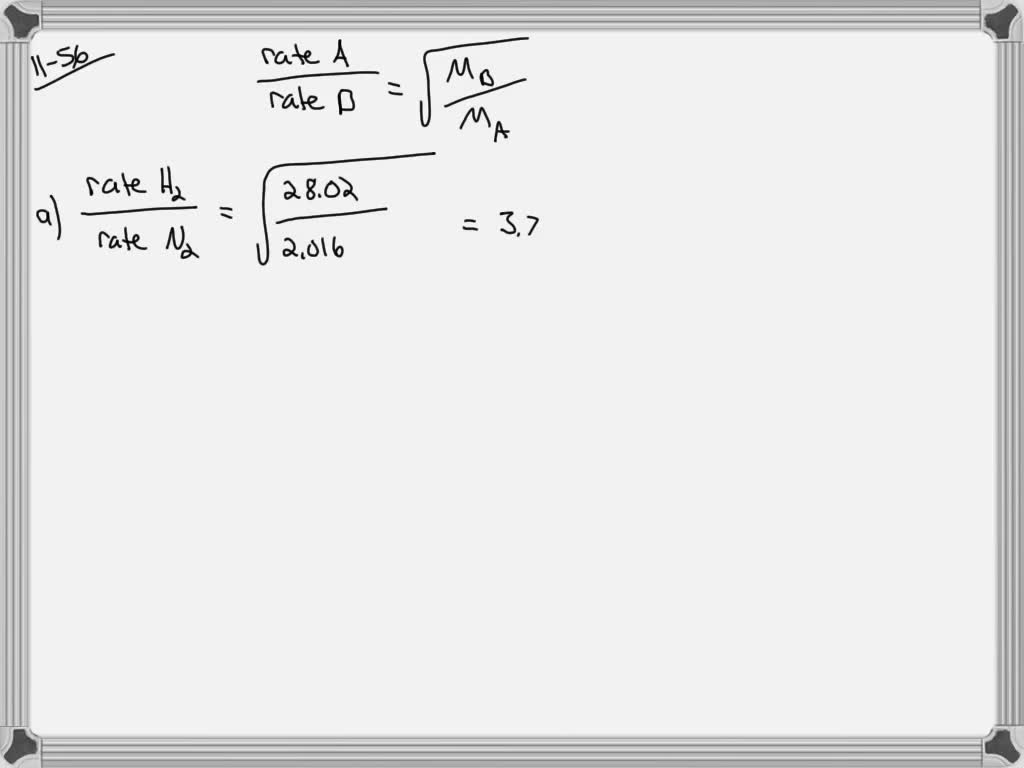
Source Image: numerade.com
Download Image
SOLVED: calculate the ratio of effusion rates for n2 and ne
⏩SOLVED:Quantitatively compare the rates of effusion for the… | Numerade Solution A Solution B Create an account to view solutions Recommended textbook solutions Pearson Chemistry Matta, Staley, Waterman, Wilbraham 3,748 solutions Chemistry: The Central Science 14th Edition Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown 7,848 solutions
Calculate the ratio of effusion rates for nitrogen $(N_2)$ a | Quizlet Quantitatively compare the rates of effusion for the followi | Quizlet By calling neon “gas 1” and argon “gas 2,” we can compare the effusion rates of the two gases by plugging their molecular masses into the equation. This proportion is equal to the rate of neon effusion over the rate of argon effusion, giving the ratio of neon atoms to argon atoms in container B.